
Human hair contains Gold (Au), Mercury (Hg), Silver (Ag) and Uranium (U) (Gellein et al 2008, Phillips et al 2010, Suárez-Criado et al 2023). The modest duckweed, a small, ubiquitous floating aquatic plant has Europium (Eu), Lutetium (Lu), Praseodymium (Pr), Thorium (Th), Thulium (Tm) and many others. Seawater as well has about any imaginable element. According to Bruland and Lohan (2003) , Indium (In) is present in the oceans at a concentration1 of 6 to 12 ppq2, Osmium (Os) at 3 to 11 ppq and Tantalium at 4 to 26 ppq. Using the slightly more familiar ppb (μg – microgrammes – per liter) this would be 0.000006 to 0.000012 for In, 0.000003 to 0.000011 for Os and 0.000004 to 0.000026 for Ta.
Geochemistry3 is the science that tracks and explains the distribution of chemical elements on earth. One could imagine that at some early stage of the earth formation, the chemical elements were more of less uniformly distributed, but then the heavier elements migrated to the core and lighter silicates (including clay minerals) came to dominate the crust. As a result of the appearance of life and photosynthesis, oxygen eventually accumulated in the atmosphere… A couple of billion years later, everything has been thoroughly mixed, eventually leading to the current situation where we have “everything everywhere”, sometimes at exceedingly low concentrations, as illustrated in the first paragraph above. But there are also local peaks or “spikes” of element concentration, as in metal deposits (ores), resulting from specific chemical and geological circumstances.
Biology too plays a part, at various scales (e.g. Killops and Killops, 2005) and organisms themselves may lead to element concentration spikes. There is not only guano – which constitutes a spike of carbon (C), nitrogen (N) and Phosphorus (P) – but there are also some well known spikes of heavy metals and other elements, for instance Mercury (Hg) in fish, Lead (Pb) in the bones of Roman skeletons, and Arsenic (As) and even Thallium (Tl) in the remains of people deliberately sent to the afterlife in Agatha Christie novels (4.5 from Paddington, The pale horse).
This post looks more specifically at honey and assesses whether it possibly constitutes a spike for some elements, after a previous post found widespread background pesticides and heavy metals.
Before focusing on chemical elements in honey, it is necessary to mention the most obvious source of those elements, i.e. mostly flowering plants. There is a good chance that at least some chemical elements that are naturally present in plants in large quantities (e.g. the above mentioned spikes) will be carried over to honey during the process of mellification4. The sections below will also suggest that bees may somehow selectively enrich honey in some elements or, on the contrary, exclude others.
1. On the special relations of certain plants with some chemical elements
Many plants are “naturally” (i.e. genetically) tolerant to toxic chemicals, such as the salt tolerant halophytes5. There are also examples of plants that acquire metal tolerance, for instance for Cadmium (Cd; Gratao et al 2008).
Plants that are able to withstand large concentrations of metals are referred to as Metallophytes. A distinction is made between ‘obligate metallophytes’ (which actually require the presence of these metals) and ‘facultative metallophytes’, which can tolerate such conditions but are not dependent on them. Many plants, and not exclusively the various shades of metallophytes, can detoxify the harmful elements by blocking them chemically or relocating them outside the cytoplasm, for instance in cell walls (for the example of Antimony6 (Sb), see Feng et al 2013; or Skuza et al 2022 for a more generic treatment7 of the subject).
Some plants are known as hyperaccumulators, i.e. they absorb large quantities of metallic elements, sometimes reaching concentrations that exceed those in the environment. The accumulation maybe an accidental side effect of some other natural physiological process but cases of active absorption have also been reported (Feng et al 2013). According to Leitenmaier and Küpper (2013) , however, active metal transport […] is the key mechanism of hyperaccumulation.
Wikipedia has a list of Hyperaccumulator plants. They are very common among aquatic plants, as already mentioned with the example of duckweed. Among land plants one of the most common group of accumulator plants is the cabbage family, including Brassica napus (rapeseed, canola). Accumulated elements include Manganese (Mn), Lead (Pb), Selenium (Se), Chromium (Cr), Copper (Cu) and Zinc (Zn) in addition to the already mentioned Silver (Ag)8. Accumulators of Arsenic (As), Aluminium (Al), Manganese (Mn), Lead (Pb) and Zinc (Zn) are common among grasses, which are also rich in Silicon (Si; the element plays a part in the structural strength of the stalk and as herbivore repellent; Kumar et al 2017, Hartley and L. DeGabriel 2016)9. Other accumulator plants that deserve mentioning include sunflower (Helianthus) for zinc (Zn), Manganese (Mn), Chromium (Cr) and Copper (Cu), clover for Zinc (Zn) and willows for Silver, Chromium, Mercury, Lead, Selenium and Zinc (Ag, Cr, Hg, Pb, Se, Zn). Willows are an important early spring source of pollen for some honeys although no readily available source of data on the listed metals in willow pollen could be located. Table 2 in Reeves et al (2017) lists important families, genera and regions of occurrence of hyperaccumulators by chemical element.
Opinions differ as to the role and amounts of heavy metals in nectar compared with the amounts found in honey. Xun et al (2018) mention that pollinators sometimes avoid plants with high heavy metal concentrations, while Borsuk et al (2021) and Tomczyk et al (2023) find that bees can more of less detoxify nectar for some elements: Iron (Fe) down 40-fold, Zing (Zn) down 26-fold, Copper (Cu) and Cadmium (Cd) down 8-fold but Lead (Pb) was unchanged. Possibly, the detoxification requires that bees “recognize” (“smell”?, “taste”?) some elements, as some research suggests for copper (Cu) and zinc (Zn)10.
A last point, which will not be examined beyond quoting a paper by Merritt and Bewick (2017), is that insects relations with heavy metals show considerable diversity across taxa. It is likely that specific bee races show themselves different behaviours and sentivities vis à vis specific elements.
2. The chemical composition of honey compared with the composition of the earth’s crust

In my previous post Les abeilles, leur miel et ce qu’on trouve dedans (“Bees, their honey and what can be found in it”) I prepared a table with heavy metals in honey taken from various sources. Some authors collect data and try to relate the chemical composition of honey considered as an “indicator” to environmental conditions; honey can indeed be used as an indicator of environmental health provided pollution is severe.
Table 1 below is a more complete version of the table numbered 3 in the first post. It is assumed that it provides somehow “representative average” values of the elemental composition of honey11. Note that all concentrations are referred to honey as harvested and sold, i.e. essentially a solution of sugars containing 10 to 20% of water. Concentrations referred to dry weight would be 5 to 10 times larger, respectively, for 80% and 90% of water in honey. Accounting for the much lower water content in the earth’s crust would leave abundances largely unchanged.
The text and the figures below are stuctured by adopting the geochemical classification of elements developed by Goldschmidt12 which groups elements into 4 categories referred to as lithophile (rock-loving), siderophile (iron-loving), chalcophile (sulfide ore-loving or chalcogen-loving), and atmophile (gas-loving) or volatile.
Table 1 presents the relative abundance of elements in the earth’s crust and in honey by Goldschmidt classes and increasing atomic weight.

The abundance of elements in the crust and in honey is also given (in ppm) as well as their relative abundance (Ratio) on a scale from -4 to 3. This scale (which is simply the base 10 logarithm of the Ratio) indicates the order of magnitude of the Ratio, which spans from 0.00003884 for Silicon to 1467 for Carbon. The colour scale is summarized in the box that precedes Table 1.

The Goldschmidt classes are examined in turn below. Several figures will be mentioned and analysed for each of them. Most of them adopt a logarithmic scale as element concentrations vary over several orders of magnitude:
- Figure 2 shows the range of concentrations using a boxplot. Except for the atmophile elements, the abundances of elements are generally about 10 to 100 times larger in the earth’s crust than in honey;
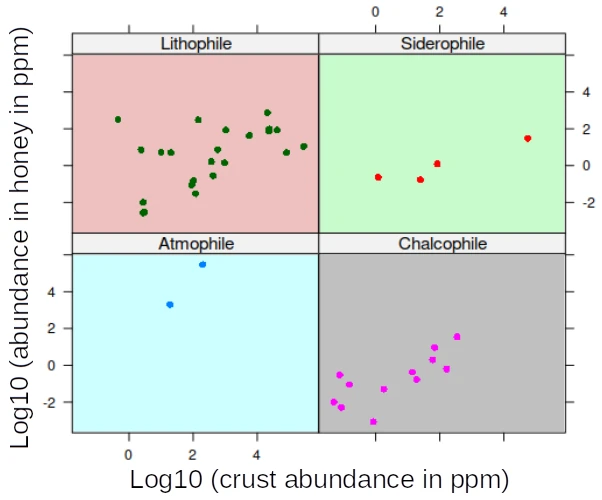
- Figure 3 and Figure 4 compare how concentrations in honey vary as a function of the concentration in the crust. All correlations are positive for all classes, but the correlation is weaker for the lithophile elements. Figure 4 includes basically the same information as Figure 3 but also identifies the elements and indicates their essentiality. There are 21 essential trace elements, i.e. elements that are essential for the the normal functioning of organisms. Some of them (marked by two asterisks in Table 3 of post 1) may nevertheless become toxic at higher concentrations. They include Manganese, Iron, Cobalt, Copper, Zinc, Selenium, Bromium, Iodine and Molybdenum (Mn, Fe, Co, Cu, Zn, Se, Br, I, Mo). The essentiality for humans is debated for Lithium, Boron, Fluorine, Slicon, Vanadium and Nickel (Li, B, F, Si, V, Ni) while the following elements are not essential for humans but for some other eucaryotes, including possibly bees14: Al, Ti, Rb, Sr, Cd and Ba (Aluminium, Titanium, Rubidium, Strontium, Cadmium and Barium). There is abundant literature on the toxicity of trace metals, including some very unusual ones (Babula et al 2008). Macro-elements, also referred to as poly-elements i.e. those that constitute the bulk of biomass (mostly Carbon C, Hydrogen H, Nitrogen N, Oxygen O, but also Phosphorus P, and Sulphur S) and the main structural inorganic components (metals such as Sodium Na, Potassium K, Magnesium Mg , Calcium Ca and the non-metallic Chlorine Cl) are not highlighted in Figure 4;
- Figure 5 shows that, in all element classes, the abundance decreases with increasing atomic weights. For lithophile and chalcophile elements, the atomic weights largely overlap (from 10 to 200) but some light lithophiles (Lithium Li and Beryllium Be, Boron B and Bromium Br) are even lighter than the atmophiles. The siderophile elements for which data are available in Table 1 are in the narrow atomic weight range from 56 to 96.
2.1 Atmophile elements
The most abundant category is that of the atmophile (volatile) elements carbon C and Nitrogen N. In combination with Hydrogen, they constitute the bulk of honey where they occur in water (with oxygen O, a lithophile element), sugars as well as some minor organic compounds (for honey!) such as fats, amino acids etc. The post on Les abeilles, leur miel et ce qu’on trouve dedans has some additional information on the minor organic compounds found in honey. They are “minor” as far as their concentration is concerned, but important in terms of honey flavour.
The abundance of Carbon and, to a lesser extent that of Nitrogen, is rather trivial in the sense that honey is an organic product. For the same reason, the abundances of the Chalcophiles Silicon (Si) and Aluminium are very low but nevertheless illustrate the ability of bees to physically and chemically separate their living environment from the mineral environment.

2.2 Chalcophile elements
In terms of their relative abundance in honey, the chalcophile15 elements come next. Most elements are metals, except for sulphur (S) and selenium (Se), which are part of the Oxygen family (group 8 of the Periodic table) and arsenic (As) , which is part of the Nitrogen (N) family (Group 7).
Their relative abundance compared with the crust is about 5% (the median is 0.04652). Those elements easily combine with sulphur and form insoluble salts. Many classical ores of the chalcophile elements are sulfides including cinnabar (mercury Hg sulfide), galena (lead Pb sulfide), realgar (arsenic As sulfide), sphalerite (zinc Zn sulfide), and pyrite (iron disulfide), and chalcopyrite (iron-copper Fe-Cu sulfide). Chalcophile elements typically occur in rich and easily extractable deposits, although they are significantly less abundant in the crust than the lithophile elements. If the data that underlie Table 1 can be trusted, it is surprising that Cadmium (Cd) and especially Silver (Ag) and, to some extent Selenium (Se) are so abundant in honey. Zinc and cadmium are close chemical relatives. It is difficult to understand why Zinc, which is about 500 times more abundant than Cadmium in the crust should be only three times more abundant in honey16. Burden et al (2019) indicate that bees can identify copper and lead (of the same chalcophile family), but not cadmium and possibly lead and zinc are excluded by bees. The least abundant chalcophile element (in relative terms) is Tl, which is probably also the most toxic. According to Wikipedia, thallium compounds have long been readily available as rat poison. This, and that it is water-soluble and nearly tasteless, led to frequent intoxication caused by accident or criminal intent.
Selenium, which behaves somehow like sulphur in biological systems is also know for it’s bio-accumulation in some organisms. A concentration of 2 ppb in water is considered toxic, and the toxic behaviour of Se is related to the one of Mercury (Hg: Spiller 2018, Raymond and Ralston 2020, Tinggi and Perkins 2022). Honey has 10 ppb but no poisoning seems to have been reported. Several papers by Whanger (starting from 1985) have studied the interactions between Selenium (Se), Cadmium (Cd), Mercury (Hg) and Silver (Ag). Whanger’s view was that selenium could counteract the toxicity of some heavy metals.

2.3 Lithophile elements
The lithophile elements, which are particularly abundant in the crust because of their affinity with oxygen, are, in relative terms, the least abundant in honey. In fact, they are about 10 times less abundant than the siderophile and the chalcophile elements.
It is striking that Aluminium (Al) and Silica (Si), both important components of rocks (including clays) are four orders of magnitude less abundant in honey. The same applies to Vanadium (V), an element, however, that is is far less ubiquitous in the crust. They are followed by both Calcium (Ca) and Manganese (Mn) which are three orders of magnitude less abundant.
The most interesting observation, however, is the behaviour of Iodine (I), Bromium (Br), Chlorine (Cl) and Boron, followed by Lithium (Li) and Phosphorus (P). Iodine is about a 1000 times more abundant in honey than in the crust, which would be a clear case of selective accumulation. Iodine, Chlorine and Bromium belong to the same family of the halogens (period 9). The fact that the concentrations of Cl and Br are also very high (about the same abundance as in the crust), raises the question whether bees preferentially accumulate halogens. The lightest element of the family, however, and possibly the most toxic, Fluorine (F) does not appear to be accumulated. It is those specific Halogens which mostly account for the departure from linearity of the plot of concentrations in honey Vs the concentration in the crust (Figures 3 and 4).
A word has to be added for four elements with comparably high relative concentrations in honey. They include Boron (B), about as abundant in honey as in the crust, Lithium (Li) and Phosphorus (P) (they were already mentioned above) as well as Potassium (K). The two monovalent metals (Lithium and Potassium) of Period 1 have little in common with the two others: Boron, a non-metal of period 5 and Phosphorus (period 7). Contrary to Boron, Phosphorus is ubiquitous in biochemistry where it plays an essential part in cell structure (including DNA) and the energy metabolism.
2.4 Siderophile elements
The four siderophile elements considered in this blog post are slightly less abundant than the chalcophile ones. Iron (Fe), the most abundant element of the four is very abundant in the crust (56300 ppm) but 1000 times less abundant in honey. The three others (Cobalt Co, Nickel Ni and Molybdenum Mo) seem to be excluded less from honey than iron, with Mo just slightly less abundant in honey than in the crust. The bees’ tolerance for the siderophiles is intermediate between the chalcophiles and the lithophiles.
3. Summary and conclusions
Chemical elements are mostly diluted in the earth’s crust and the environment, but there are some “spikes” of locally higher concentration e.g. in ores but also in some plants. Since bees harvest the raw materials and ingredients for honey mostly from plants, it is important to look at the abundance of elements in plants.
The blogpost lists many accumulator and even “hyperacumulator” plants for a range of elements. Opinions differ as to the role and amounts of elements, including toxic heavy metals in different plant organs and secretions, especially pollen and nectar. Considering the low concentrations in honey compared with the amounts found in some plants, there is evidence that bees can somehow detoxify the ingredients that will eventually constitute the honey.
The presentation of the results of the abundance of elements in honey folllows Goldschmidt’s geochemical classification, which starts with the atmophile (or “gas-loving”) elements, essentially Carbon (C) and Nitrogen (N) which are three orders of magnitude more abundant in biological products such as honey than in the crust.
The second category, the chalcophile elements are sulfide ore loving and include, next to Sulfur itself, several metals and non-metals chemically related to Sulfur. The elements are 10 to 100 times less abundant in honey than in the crust. There are, however, two surprises: silver (Ag) which bees concentrate 10 times relative to the crust, and Cadmium (Cd), which is at the same level as the background. Sulfur and especially Selenium have been hypothesized to play a part in the detoxification of some common heavy metals.
As to the lithophile (rock-loving) elements, they are less abundant than the chalcophiles (about 10 times). The ratio even drops to 10000 times less abundant for the typical rock components Silicon (Si) and Aluminium (Al) and for Vanadium (V). The most striking observation, however, is the behaviour of the halogens Iodine (I), Bromium (Br), Chlorine (Cl). They are all at least as abundant in honey as in the crust, and for Iodine the enrichment factor reaches 1000. This raises the question whether bees preferentially accumulate halogens.
The last class (“iron-loving” siderophiles) contains only 4 chemically similar elements, including Iron (Fe). The bees tolerance for the siderophiles is intermediate between the chalcophiles and the lithophiles.
3. Acknowlegements
Thanks to Philippe Greisch, my former primary school companion, for suggesting some welcome (as well as needed) improvements to the spelling, language and style.
4. References
Babula (Petr), Vojtech Adam, Radka Opatrilova, Josef Zehnalek, Ladislav Havel, Rene Kizek 2008 Uncommon heavy metals, metalloids and their plant toxicity: a review. Environ Chem Lett 6:189–213. https://link.springer.com/article/10.1007/s10311-008-0159-9
Bogdanov (Stephan) , Tomislav Jurendic , Robert Sieber, Peter Gallmann 2013 Honey for Nutrition and Health: A Review. Journal of the American College of Nutrition, 27:6, 677-689, https://www.researchgate.net/publication/23803275_Honey_for_Nutrition_and_Health_A_Review.
Borsuk (Grzegorz) , Aneta Sulborska , Ernest stawiarz, Krzysztof olszewski1, Dariusz wiącek, Noor ramzi, Agnieszka Nawrocka, Małgorzata Jędryczk 2021 Capacity of honeybees to remove heavy metals from nectar and excrete the contaminants from their bodies. Apidologie 52:1098–1111. https://link.springer.com/article/10.1007/s13592-021-00890-6.
Brooks RR, J Holzbecher, DE Ryan 1981 Horsetails (Equisetum) as indirect indicators of gold mineralization. Journal of Geochemical Exploration, 16:21-26. https://www.sciencedirect.com/science/article/abs/pii/0375674281901229
Bruland (Kenneth), Maeve Lohan 2003 Controls of Trace Metals in Seawater. Chapter 6.0.2 (p. 23-47) in H.D. Holland and K.K. Turekian (Editors), Treatise on Geochemistry, 1st edition. https://www.whoi.edu/cms/files/BrulandLohan_TOG_52025.pdf. There is a second edition (2014) where Controls of Trace Metals in Seawater is apparently p. 19-51 in volume 8 and the authors are Kenneth Bruland, Rob Middag & Maeve Lohan. See here for the 2nd edition. The numbers quoted in this post are from the 1st edition, which is freely available on the web.
Burden (Christina M) , Mira O Morgan, Kristen R Hladun, Gro V Amdam, John J Trumble, Brian H Smith 2019 Acute sublethal exposure to toxic heavy metals alters honey bee (Apis mellifera) feeding behavior. Scientific Reports 9:4253. OpenAccess paper.
dos Santos Scholz (Maria Brı́gida), Alécio Quinhone Júnior, Bruna Haas Delamuta, Jessika Marie Nakamura, Marianne Cristina Baudraz, Mônica Oliveira Reis, Talita Kato, Mayka Reghiany Pedrão, Lucia Felicidade Dias, Dalton Tadeu Reynaud dos Santos, Cintia Sorane Good Kitzberger, Fabrı́cio Pires Bianchini 2020 Indication of the geographical origin of honey using its physicochemical characteristics and multivariate analysis. J Food Sci Technol 57(5):1896–1903. https://link.springer.com/article/10.1007/s13197-019-04225-3
Fehlauer (Till), Blanche Collin, Bernard Angeletti, Mohammad Mustafa Negahi, Cédric Dentant, Perrine Chaurand, Claire Lallemand, Clement Levard, Jérôme Rose 2022 Multiscale imaging on Saxifraga paniculata provides new insights into yttrium uptake by plants. Nature Sci Rep 12, 18268. https://doi.org/10.1038/s41598-022-23107-x
Feng (Renwei), Chaoyang Wei, Shuxin Tu, Yongzhen Ding, Ruigang Wang, Junkang Guo 2013 The uptake and detoxification of antimony by plants: A review. Environmental and Experimental Botany 96:28–34. https://www.sciencedirect.com/science/article/abs/pii/S0098847213001214.
Flamminii (Federica) , Ada Consalvo, Angelo Cichelli, Alessandro Chiaudani 2024 Assessing Mineral Content and Heavy Metal Exposure in Abruzzo Honey and Bee Pollen from Different Anthropic Areas. Foods 13:1930. https://doi.org/10.3390/foods13121930
Garten (Charles T Jr) 1986 Correlations between concentrations of elements in plants. Nature 261:686-688. https://www.nature.com/articles/261686a0
Gellein (Kristin), Syverin Lierhagen, Per Steinar Brevik, Marte Teigen, Parvinder Kaur, Tajeshwar Singh, Peder Flaten Trond, Tore Syversen 2008 Trace element profiles in single strands of human hair determined by HR-ICP-MS. Biol Trace Elem Res. 123(1-3):250-60. https://pubmed.ncbi.nlm.nih.gov/18286238.
Gratão PL, CC Monteiro, AM Antunes, LEP Peres, RA Azevedo 2008 Acquired tolerance of tomato (Lycopersicon esculentum cv. Micro-Tom) plants to cadmium-induced stress. Ann Appl Biol 153:321–333. https://onlinelibrary.wiley.com/doi/10.1111/j.1744-7348.2008.00299.x
Hartley (Susan E.), Jane L. DeGabriel 2016 The ecology of herbivore-induced silicon defences in grasses. Functional Ecology 30:1311–1322. https://besjournals.onlinelibrary.wiley.com/doi/epdf/10.1111/1365-2435.12706
Herzig, Rolf & Rehnert, Albert & Korhammer, Siegfried & Kumpulainen, Jorma & Schramel, Peter & Muntau, Herbert & Linsinger, Thomas & Quevauviller, Philippe. (2002). Certification of a new cabbage reference material for the quality control of trace-element determinations with some considerations on moisture. TrAC Trends in Analytical Chemistry 21(11):746-761. https://www.sciencedirect.com/science/article/abs/pii/S0165993602011032
Killops (Stephen D), Vanessa J Killops 2005 Introduction to organic geochemistry. Blackwell Publishing Company, 393 pp. https://www.geokniga.org/bookfiles/geokniga-introductiontoorganicgeochemistry.pdf
Krull (Elaine S) 2019 Calculation of Nitrogen-to-Protein Conversion Factors: A Review with a Focus on Soy Protein. J Am Oil Chem Soc. 96(4):339-364. https://www.scribd.com/document/800525880/J-Americ-Oil-Chem-Soc-2019-Krul-Calculation-of-Nitrogen-to-Protein-Conversion-Factors-a-Review-With-a-Focus-on-Soy
Kumar (Santosh), Milan Soukup, Rivka Elbaum 2017 Silicification in Grasses: Variation between Different Cell Types. Front Plant Sci 8:438. https://pubmed.ncbi.nlm.nih.gov/28400787/
Leitenmaier (Barbara), Hendrik Küpper 2013 Compartmentation and complexation of metals in hyperaccumulator plants. Frontiers in Plant Science, 4, 13 pp. https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2013.00374/full
Merritt (Thomas J.S.), Adam J. Bewick 2017 Genetic Diversity in Insect Metal Tolerance. Front. Genet. 8:172.https://pmc.ncbi.nlm.nih.gov/articles/PMC5673992/pdf/fgene-08-00172.pdf
Nkrumah (Philip Nti ), Antony van der Ent 2023 Possible accumulation of critical metals in plants that hyperaccumulate their chemical analogues. Science of The Total Environment, 878, article 162971. https://www.sciencedirect.com/science/article/abs/pii/S0048969723014079
Pauwels (Jean), Andrée Lamberty, Heinz Schimmel 1998 The determination of the uncertainty of reference materials certified by laboratory intercomparison. Accred Qual Assur 3:180-184. https://www.researchgate.net/publication/225737941_The_determination_of_the_uncertainty_of_reference_materials_certified_by_laboratory_intercomparison
Phillips (Genevieve), Frank Reith, Clifford Qualls, Abdul-Mehdi Ali, Mike Spilde, Otto Sppenzeller 2010 Bacterial Deposition of Gold on Hair: Archeological, Forensic and Toxicological Implications. PLOS ONE 5(2): e9335. https://doi.org/10.1371/journal.pone.0009335
Quevauviller, Philippe & Fortunati, Umberto & Vercoutere, Kristien & Muntau, Herbert & Maier, E. & Griepink, Bernard. (1996). Certified reference materials of soils and sewage sludges for the quality control of trace element environmental control. TrAC Trends in Analytical Chemistry. 15. 504-513. https://www.sciencedirect.com/science/article/abs/pii/S0165993696807394
Raymond (Laura J), Nicholas V C Ralston 2020 Mercury: selenium interactions and health implications. NeuroToxicology 81:294-299. https://www.sciencedirect.com/science/article/pii/S0161813X20301546
Reeves (Roger D.), Alan J.M. Baker,Tanguy Jaffré, Peter D. Erskine, Guillaume Echevarria, Antony van der Ent 2017 A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytologist 218:407–411. https://nph.onlinelibrary.wiley.com/doi/epdf/10.1111/nph.14907
Solayman (Mohammed) , Md Asiful Islam, Sudip Paul, Yousuf Ali, Md Ibrahim Khalil, Nadia Alam, Siew Hua Gan 2016 Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins: a comprehensive overview. Comprehensive Reviews in Food Science and Food Safety 15(1):219-233. https://pubmed.ncbi.nlm.nih.gov/33371579/
Spiller (Henry A) 2018 Rethinking mercury: the role of selenium in the pathophysiology of mercury toxicity. Clin Toxicol (Phila) 56(5):313-326. https://pubmed.ncbi.nlm.nih.gov/29124976/
Suárez-Criado (Laura), Pablo Rodríguez-González, José Marrugo-Negrete, J. Ignacio García Alonso, Sergi Díez 2023 Determination of methylmercury and inorganic mercury in human hair samples of individuals from Colombian gold mining regions by double spiking isotope dilution and GC-ICP-MS. Environmental Research Volume 231, Part 1, 115970. https://www.sciencedirect.com/science/article/pii/S0013935123007624/pdfft?md5=cf370bdec69f4eea093688becbdf2389&pid=1-s2.0-S0013935123007624-main.pdf
Skuza (Lidia), Izabela Szućko-Kociuba, Ewa Filip, Izabela Bożek 2022 Review: Natural Molecular Mechanisms of Plant Hyperaccumulation and Hypertolerance towards Heavy Metals. Int. J. Mol. Sci. 23:9335. https://www.mdpi.com/1422-0067/23/16/9335
Tinggi (Ujang), Antony V Perkins 2022 Selenium Status: Its Interactions with Dietary Mercury Exposure and Implications in Human Health. Nutrients 14(24):5308. https://pmc.ncbi.nlm.nih.gov/articles/PMC9785339/
Tomczyk (Monika), Grzegorz Zaguła, Mateusz Kaczmarski, Czesław Puchalski, Małgorzata Dżugan 2023 The Negligible Effect of Toxic Metal Accumulation in the Flowers of Melliferous Plants on the Mineral Composition of Monofloral Honeys. Agriculture 13(2): 273. https://www.mdpi.com/2077-0472/13/2/273
Wang J, QX Li 2011 Chemical composition, characterization, and differentiation of honey botanical and geographical origins. Adv Food Nutr Res. 62:89-137. https://www.sciencedirect.com/science/article/abs/pii/B978012385989100003X?via%3Dihub
Whanger (Philip D) 1985 Metabolic Interactions of Selenium with Cadmium, Mercury, and Silver. In: Draper, H.H. (eds) Advances in Nutritional Research. Advances in Nutritional Research, vol 7. Springer, Boston, MA. https://doi.org/10.1007/978-1-4613-2529-1_9https://link.springer.com/chapter/10.1007/978-1-4613-2529-1_
Xun (Erna), Yanwen Zhang, Jimin Zhao, Jixun Guo 2018 Heavy metals in nectar modify behaviors of pollinators and nectar robbers: Consequences for plant fitness. Environmental Pollution 242:1166-1175. https://www.sciencedirect.com/science/article/abs/pii/S0269749117353848.
Zhou (Xiaoteng), Mark Patrick Taylor, Helen Salouros, Shiva Prasad 2018 Authenticity and geographic origin of global honeys determined using carbon isotope ratios and trace elements. Nature Scientific Reports 8(1):114639. https://www.nature.com/articles/s41598-018-32764-w.pdf
5. Notes
- The terms Concentration and Abundance seem to be used interchangeably in the geochemical practice. ↩︎
- I had to dig deep into my knowledge of units and chemistry to work that out. 1 ppq is 1 part per quadrillion (billionth of billionth), i.e. the millionth part of a ppb which expresses a concentration of 1
μg (microgramme) per liter (or Kg). In addition, Bruland and his colleagues use molarities. For instance, the concentration of Indium is given as 40 to 100 fmol per Kg, an fmol (or femtomole) being 10-15 mole. I was able to verify my spreadsheet calculation with this website, the only one among many that is able to handle the “extreme” units given by Bruland. ↩︎ - Wikipedia has a much less friendly and more ambitious definition: Geochemistry is the science that uses the tools and principles of chemistry to explain the mechanisms behind major geological systems such as the Earth’s crust and its oceans. The Wikipedia definition ignores the fact that there is much more to geochemistry than chemistry; there is also geology, and geophysics (e.g. the atmosphere), and living organisms… which add a lot of colour… ↩︎
- According to the Wiktionary, the word has the twofold meaning of (1) the production of honey by honey bees and (2) the preservation of something in honey. ↩︎
- I have observed on many occasions that bees themselves prefer the slightly saline water (about 3 or 4 g of salt per liter) of a salt water pool to pure water. ↩︎
- Several common plants have been identified as antimony hyperaccumulators, including narrowleaf plantain (up to 1150 ppm in the roots), and Silene vulgaris (1164 ppm in the above-ground parts) (Feng et al 2013). ↩︎
- Skuza and her colleagues list the following mechanisms of metal resistance: the formation of symbioses with rhizosphere microorganisms, the secretion of substances into the soil and metal immobilization, cell wall modification, changes in the expression of genes encoding heavy metal transporters, heavy metal ion chelation, and sequestration, and regenerative heat-shock protein production. ↩︎
- It is not surprising that cabbage was among the first plants to be proposed as standard reference materials (Herzig et al 2002). It is also stressed that rapeseed honey is one of the common mono-floral honeys. I would love to see specific trace element analyses of rapeseed honey. ↩︎
- Just for the anecdote, as the plant has probably no relevance for honey and bees (beyond honey in horsetail infusions!): horsetail (Equisetum) has been known since the late 1800s as an accumulator of Silica (Silicon dioxide) and Gold (Au). Ashes contain up to 42% of Si, which plays a more clearly structural role than in grasses. Gold (up to 10 ppm) is sometimes accumulated more than 100-fold compared with soil (Brooks et al 1981). ↩︎
- Heavy metal concentrations in nectar or pollen are often larger than the ones in honey, which indicates that bees can get rid of the metals.The specific case of Zn and Cu Vs. Cd is mentioned by Burden et al 2019. ↩︎
- There are many studies that attempt to determine the origin of honey based on its chemical composition, mainly organic ones (dos Santos Scholz et al 2020, Qand & Li 2011) or a combination of organic and mineral components (Zhou et al 2018). The latter authors, for instance state that (text modified and simplified) there were significant differences between honey samples from mainland Australia and Tasmania in the trace Ca, Mg and Sr. Commercial honey samples from mainland Australia and overseas (Asia, Europe and North America) had significant differences in the trace elements Ba, Ca, Fe, Mn, P, Na and Sr. However, Tasmania-Asia and Europe and Asia-Europe showed significant differences in the trace elements Fe and P. Although the differences are no doubt real and significant, they are nevertheless anecdotal, i.e. there are no evident factors that account for the differences between, e.g. “Australia” Vs “overseas (Asia, Europe and North America)“. ↩︎
- Goldschmidt’s classes are based mostly on “mineral” chemistry. Many elements with similar properties tend to occur together and behave similarly on biological systems as well. One of the most typical is divalent metals (e.g. Calcium or Magnesium) with Zn and Cd. Nrumah and van der Ent (2023) assume that halophytes, which absorb sodium (Na) have the potential to accumulate the less common but more “valuable” Lithium (Li), a close relative of sodium (Na). Early studies, possibly starting with Garten (1976) highlighted correlations between chemical elements, for instance Ca-Mg. It is not always clear, however, why some elements are correlated. For instance, Fehlauer et al (2022) note very significant positive correlations in a Saxifraga species between Calcium (Ca), Yttrium (Y) and Cerium (Ce) and negative ones between the same elements and Phosphorus (P). ↩︎
- The values provided by this author (Wergosum) are those for C and N. Carbon was derived from the carbon content in sugars, fats and proteins; the bulk of N in honey was assumed to be confined to proteins. Average concentrations for C and N were taken from one or more of Krul (2019), Sage-tips and bns-institute. ↩︎
- Eucaryotes (the word means “true nucleus”) include basically all organisms (including unicellular ones and fungi) except bacteria. ↩︎
- From a Greek root meaning copper. ↩︎
- One of the reasons why zinc and cadmium are so popular among heavy metals studies is that they are easily detected using atomic absorption spectroscopy. L’outil crée la fonction, et la mode, et parfois le problème… ↩︎