
Explaining the title
This post is about the somewhat puzzling observation that decaying Laurustine leaves (Viburnum tinus L.) can spontaneously inflate, i.e. fill with gas and become buoyant. I am very proud of the title “Anoxic utrification of Laurustine (Viburnum tinus L.) leaves” but I must admit that the word utrification was invented2 for the purpose of this blogpost. It is based on the Latin word uter which has several meanings, including “leather pouch” or “bag”, like the bag of a bagpipe or the leather canteens still used by sheperds around the Mediterranean. Therefore, utrification means “becoming an uter” or “transforming into an uter“3. Anoxic, which will be explained in more detail below, means “without oxygen”, and characterises the putrid mud that often results from the decomposition of organic material in stagnant water.
How it happened
In my garden there is a large Laurustine which I have referred to in the different context of this post.
I regularly collect dead leaves and compost them with other organic garden and kitchen waste, but some inevitably end up in one of the nearby drains, which have been described in detail in another post4.
When I recently cleaned the drains, I found half a dozen of “objects” floating at the surface of the water. The “objects” all had a dark face and a translucent face, which is clearly visible in Figure 1B. After cleaning them it was rather evident that they were Viburnum leaves, as shown in Figure 1C. I first hesitated piercing the “bladders” because I thought I was dealing with some animal5, but when I finally did, I found nothing but “air” and some water inside (Figure 1D).

The process of organic material decomposition in water
I wrote “air” above but the gas filling the leaves is unlikely to be air. The black colour of the mud clearly points at the lack of oxygen, i.e. anoxic conditions, which often develops when organic matters decays in water. The colour derives from very soluble reduced iron (Fe2+); on the contrary, its oxidized counterpart which occurs in oxygen-rich environments (Fe3+) is colloidal and reddish, i.e. rust-coloured.
When leaves decay in air (i.e. an oxygen-rich environment), the process produces a number of chemicals, but the main gas produced is the oxygen-rich carbon dioxide CO2. When the process occurs in water, the micro-organisms degrading organic matter rapidly deplete the available oxygen, after which other microorganisms take over and the gases produced include mostly methane CH4 and hydrogen sulphide H2S, a gas that humans perceive as very unpleasant even at low concentrations and which is one of the main ingredients of the odour of rotten eggs and sewers6. The phenomenon is well known but, amazingly, there seems to be little information about the dynamics (amounts, relative timing) of methane and hydrogen sulphide production7. The references mention a 1984 paper by Avnumelech et al. and a 2015 paper by Liu et al. The Liu paper has some quantitative data. Under laboratory conditions, the authors show that sewer sediment initially produces relatively more sulphide but that, in general, sulphide and methane are produced in parallel over long periods. The data in the note also indicate that methane is produced in the most reducing environments.
In all likelihood, the gas that inflates the Viburnum leaves is mostly methane. I had first assumed that the gas was “a mix of hydrogen sulphide and probably some methane” but the first commenter (below; also see the paper by Bauduin, Gypens and Borges, 2024) tells me that (1) terrestrial environments contains little sulphate (which is assumed to be the source of sulphur for H2S) and that, (2) due to its high solubility, gazeous H2S is unlikely to be present in any significant amount8.
For the surface of the leaves to survive the decomposition process, they must be more resistant to decay than other components9. The outermost layer of leaves is typically a fatty-waxy cuticle which is one of the reasons why many leaves don’t wet. We can only guess the detail of the processes involved. One of the main functions of the cuticle is to prevent excessive water loss by plant organs, but the cuticle is normally pierced with small holes (known as stoma, plural: stomata) that allow the exchange of oxygen and carbon dioxide used/produced in the processes of respiration and photosynthesis. In order for the leaves to be able to inflate, it must be assumed that the stomata are somehow obturated, or possibly the production of gas inside the leaves exceeds losses.
There is an additional possible inflation mechanism, depending on the nature of the layer bounding the leaves: osmosis. If the concentration of dissolved substances inside the leave exceeds that of the water in which the leaves are decaying, this will lead to water entering the leaf and inflating it, but it does not account for the presence of gases.
Finally, it is interesting to observe (Figure 1B) that one of the sides of the leaves is very thin and parchment-like as well as translucent, while the other is black and muddy. It is very likely that the dark and muddy side of the leaf was in contact with the mud deposit in the sewer (refer, again, to P in Figure 2 of the sterput post), while the other was in contact with water or air. As the water in the substrate tends to evaporate, the deposit goes through phases of wetting and drying, with the result that the face of the leaf facing upward10 goes through drying and re-wetting, while the lower part remains mostly wet.
If the gas is indeed generated inside the leaf, it is likely that it squeezes out the water, through the lower face of the leave, the one which is provides with stomata. Once there remains no liquid, I assume the leaf deflates.
Conclusion
Decaying Laurustine leaves (Viburnum) can fill with gas and some liquid under anoxic conditions and become buoyant. The process is referred to in the title as utrification. It is not clear whether other leaves would behave similarly but it should be relatively easy to reproduce the phenomenon experimentally. Some questions to be answered would include the timing of the phenomenon and whether leaf orientation (lower side up or down, i.e. in contact with the water or the sediment) plays a part. If I had a chemistry lab with a gas-chromatographer, I would also love to know the composition of the gases inside the inflated leaves11.
References
Avnimelech Y, McHenry JR, Ross D 1984 Decomposition of Organic Matter in Lake Sediments. Environ. Sci. Technol. 18(1), Vol. 18(1):5-11.
Bauduin T, Gypens N, Borges AV 2024 Methane, carbon dioxide and nitrous oxide emissions from two clear-water and two turbid-water urban ponds in Brussels (Belgium), EGUsphere [preprint], https://doi.org/10.5194/egusphere-2024-1315, 2024.
Keeny DR, Herbert RA, Holding AJ 1971 Microbiological Aspects of the Pollution of Fresh Water with Inorganic Nutrients, pages 181-200 in: G Sykes and FA Skinner. Microbial Aspects of Pollution, Academic Press, London.
Knols BGJ, De Jong R 1996 Limburger cheese as an attractant for the malaria mosquito Anopheles gambiae s.s. Parasitology Today, 12(4), 159–161.
Knols BG 1996 On human odour, malaria mosquitoes, and Limburger cheese. The Lancet, 348(9037), 1322.
Knols BGJ, van Loon JJA, Cork A, Robinson RD, Adam W, Meijerink J, De Jong R, Takken W 1997 Behavioural and electrophysiological responses of the female malaria mosquito Anopheles gambiae (Diptera: Culicidae) to Limburger cheese volatiles. Bulletin of Entomological Research, 87(02), 151.
Liu Y, Ni B-J, Ganigué R, Werner U, Sharma KR, Yuan Z 2015 Sulfide and methane production in sewer sediments, Water Research 70(1):350-359.
Manning DJ, Chapman HR, Hosking ZD 1976 The production of sulphur compounds in Cheddar cheese and their significance in flavour development. Journal of Dairy Research, 43(02), 313.
Notes
- It could also be due, though, to black volcanic ash, black sand or fine organic mud. ↩︎
- I have invented several “scientific” words, for instance “barometallic” (or sometimes “barymetallic”) for “pertaining to heavy metals” (from Greek baro-, weight, as in isobar, barycentre or barometer!). When the derivation is obvious, the word looks “natural” and no-one notices, including reviewers who all long only for one things: get rid of this unnecessary paper and do something more interesting. Of course, I would never invent a bizarre word like Cyndiniques which English rightly never adopted, preferring Risk management. ↩︎
- Botanists frequently use the term ŭtrĭcŭlus, which means “small uter“. The english word utricle is not uncommon in animal, including human, anatomy and botany where it is used with several meanings, from algae to flowering plant. It can be found in most English language dictionaries such as Collins, Merriam-Webster or Cambridge dictionary with slightly different acceptions. The Latin dictionary indicates that the uterus is ŭtĕr/ŭtĕri (II declension) while “soft leather bottle” is ŭtĕr/ŭtĕris (III declension). The word also specifically means floats for crossing a river and is thus excatly the word we need in the current context. In the genus Utricularia (Bladderworts), a group of floating carnivorous plants, the utricles apparently mostly serve the purpose of capturing preys. The air filled sacs in certain seaweeds that keeps the plants “upright” are mostly referred to as pneumatocyst. ↩︎
- Sterput is the francophone-Belgian variant of the Flemish word sterfput, which originally means “death well”, i.e. an infiltration hole in the ground where run-off water “dies”. The meaning of the word has evolved to design floor sinks and various types of indoor and outdoor drains. ↩︎
- In particular Hoverfly larvae, which have a soft body with a “tail” ↩︎
- I assume most of the sulphur present in plant material comes from the amino-acids methionine and cysteine. Sulphur does not seem to play any part in the leaf cuticles. ↩︎
- Some old lectures notes of mine provide a simple table (based on Keeney et al., 1971) of the processes that take place with increasing anaeroby or increasing reduction potential:
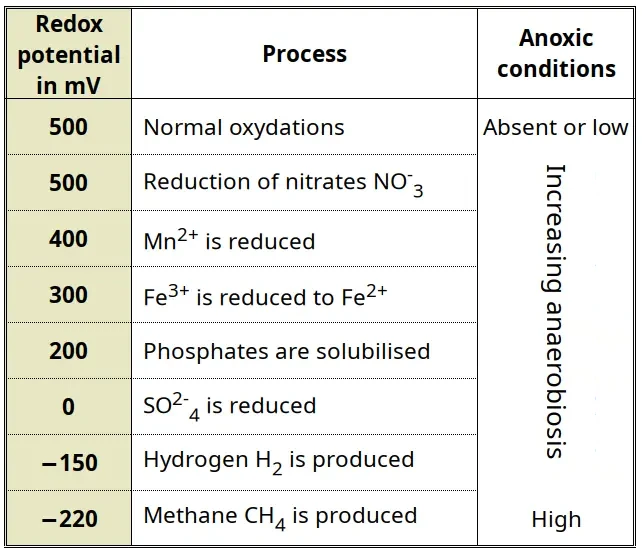 ↩︎
↩︎ - Consider, however, that Laurustine is also known as the stinking Viburnum; refer to this link and this one. One commenter in the first link compares the stench to “dogs’ muck” but to me it is much more like seasoned cheese or… feet. This typical smell is apparently due to propionic acid (Knols 1996, Knols et al. 1995, Knols et al. 1997) but also to sulphur-based components such as methanethiol and dimethyl sulphide (Manning et al. 1976). The origin of the unpleasant odour of Laurustine is definitely worth exploring as sulphur is not unlikely to be involved. ↩︎
- Viburnum leaves are often referred to a “leathery” (e.g. here, or here for Viburnum tinus). There is even a species known as Leatherleaf Viburnum. ↩︎
- This can be the upper side of the leaf, i.e. the side facing the stem, or the lower face. The dark face of the leave is the one where veins (nervatures) are most visible, while the translucent part is even and smooth. Nervatures are often anatomically closer to the lower side of the leaves. This is very visible in figure 1D. ↩︎
- The gas could easily be sampled with a small syringe. If any reader should have an idea about how to analyse the gaz in my kitchen, I’d be happy to have the info! ↩︎
A mon avis les feuilles se remplissent de CH4 et non pas de H2S: le H2S est relativement rare en milieur terrestre car il y très peu de SO42- (sulfate qui sera réduit en H2S) dans les eaux de rivières, les eaux de pluie, ou dans les sols. Le H2S est extremement soluble et restera dissout dans l’eau interstielle de la boue, et n’a pas vocation à former des bulles. Par contre le CH4 est très peu soluble dans l’eau et par conséquent forme des bulles très facilement. Celles-ci migrent ensuite vers le haut par poussée d’archimède.
Les feuilles de Viburnum vont fonctionner comme un petit entonnoir fermé qui va piéger les bulles et lorsque le tout aura assez de portance cela va remonter vers la surface.
La formation de bulles dans des mares est très bien connue et quantifiée. Coincidence du calendrier: mon equipe vient de publier un article sur le sujet. Le pdf est en libre accès.
Bien à vous
Alberto Borges
Many thanks for your comments! I have built them into the post by downplaying the role of H2S. As to how the leaves become buyant, my first guess is that the gas (methane as you suggest) is produced inside the leaves themselves.